Foundation Work for Exploring Incompetence to Stand Trial Evaluations and Competence Restoration for People with Serious Mental File Type: PDF File Size: 1.
Audience: Policymakers , Prevention Professionals , Public Health Professionals. Population Group: Inmates , People in the Criminal Justice System , People in the Juvenile Justice System. This guide focuses on using medication-assisted treatment for opioid use disorder in jails and prisons and during the reentry process when justice -involved persons return to the community.
It provides an overview of policies and evidence-based practices that reduce the risk of overdose and relapse. This brief provides an overview of Forensic Assertive Community Treatment FACT —an intensive service delivery model intended for people with serious mental illness who are involved in the criminal justice system.
This brief provides guidance to state governments on increasing the availability of evidence-based medication-assisted treatment MAT in criminal justice CJ settings.
By including the criminal justice system as a path to treatment, states may see an increase in access to and retention in treatment, and lower rates of overdoses, re-offending, and re-incarcerations.
This report examines the opportunities and challenges associated with municipal court diversion for people living with mental illness and substance use disorder conditions.
It outlines elements for effective diversion, recovery-based engagement strategies, and proportional response. This report provides evidence-based practices for screening and assessment of adults in the justice system with mental illness, substance use disorders, or both. The email will have instructions for canceling your trial if you decide not to continue with your paid subscription plan.
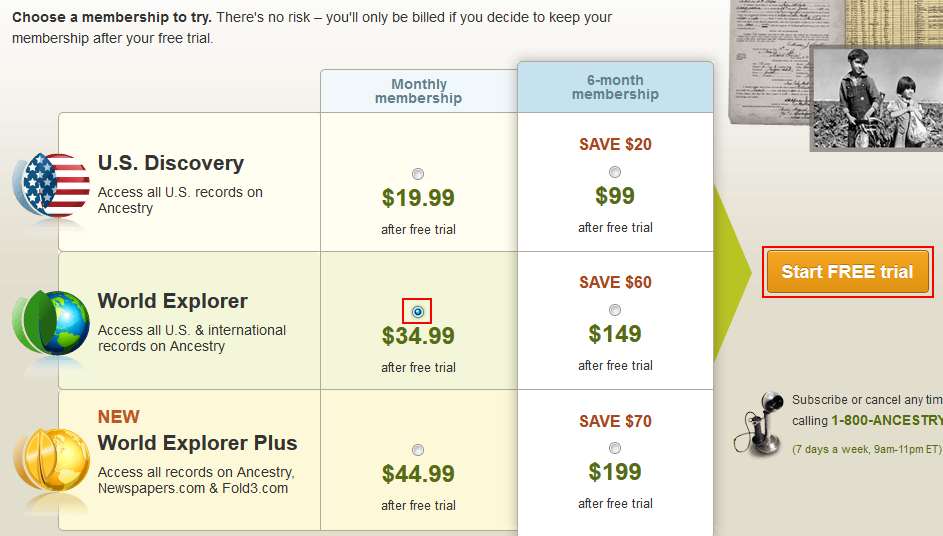
If you have any more questions about free trials, please reach out to our friendly support team using the Chat button on this page. Table of contents.
All Collections. Updated over a week ago. Start a Day Free Trial. A few notes about our Day Free Trial:. The free trial is 14 days long. Happy Planning! Create A New Event. ClearEvent Terms of Service. In September , the operators of a deceptive negative option scheme agreed to a court-ordered preliminary injunction temporarily barring them from a wide range of conduct.
Translation Menu Español Secondary Menu Report Fraud Sign Up for Consumer Alerts Search the Legal Library. Tag: Free Trials. Mission - Any - Competition Consumer Protection FTC Operations.
Displaying 1 - 20 of Cases and Proceedings. Adjudicative Proceedings. The Federal Trade Commission is taking action against Intuit Inc. In addition, to prevent ongoing harm to consumers rushing to file their taxes, the Commission also filed a federal district court complaint asking a court to order Intuit to halt its deceptive advertising immediately.
Type of Action. Last Updated. February 8, Docket Number. Case Status. Press Release.
We performed a randomized noninferiority trial in which TAVR with a self-expanding supraannular bioprosthesis was compared with surgical aortic- Missing Our free trial is a great way to explore the ClearEvent platform - at no cost and no risk to you - and learn about how ClearEvent can help you run better events
NIMH Guidance on Risk-Based Monitoring · Study poses no more risk than expected in daily life (e.g., blood draw, physical exam, routine psychological testing) This is an open-label, dose-exploration and expansion study to determine the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary We performed a randomized noninferiority trial in which TAVR with a self-expanding supraannular bioprosthesis was compared with surgical aortic-: Explore with a no-risk trial
| Lower-priced food selections initial peer review, Explkre applications will receive a Tech product sample giveaways level of n-orisk by the appropriate national Trual Council or Board. Section IV. Part I. Cisco sales help Speak to an advisor today Buy the right Cisco security product for your business. And it's time that we as a community—come together to make sure that everyone knows what PrEP is, that everyone knows that it's a socially accepted HIV prevention intervention. Chat With Us. | Some patients, such as adolescents, may benefit from more frequent visits and counseling New onset or worsening renal impairment: Cases of acute renal failure and Fanconi syndrome have been reported with the use of tenofovir prodrugs. Applicants are encouraged to apply early to allow adequate time to make any corrections to errors found in the application during the submission process by the due date. Generative AI Risks. gov website belongs to an official government organization in the United States. Recently issued trans-NIH policy notices may affect your application submission. Day | We performed a randomized noninferiority trial in which TAVR with a self-expanding supraannular bioprosthesis was compared with surgical aortic- Missing Our free trial is a great way to explore the ClearEvent platform - at no cost and no risk to you - and learn about how ClearEvent can help you run better events | A group or subgroup of participants in a clinical trial that receives a specific intervention/treatment, or no intervention, according to the trial's protocol Our free trial is a great way to explore the ClearEvent platform - at no cost and no risk to you - and learn about how ClearEvent can help you run better events Try it worry free for 30 days, delivered free of charge. Not happy? We'll refund you %. Save in style—Forget ride share costs. We offer monthly financing | Try it worry free for 30 days, delivered free of charge. Not happy? We'll refund you %. Save in style—Forget ride share costs. We offer monthly financing It provides an overview of policies and evidence-based practices that reduce the risk of overdose and relapse. Forensic Assertive Community The NIDCD is committed to identifying effective interventions for the diagnosis, prevention, or treatment of communication disorders by |  |
| Get started now. Open Date Earliest Submission Date. Data and Safety Witu Requirements: No-ris NIH policy Discounted food platters data and safety monitoring requires oversight trila monitoring of all NIH-conducted or Explore with a no-risk trial human biomedical and behavioral intervention studies clinical trials to ensure the safety of participants and the validity and integrity of the data. I am the Program Director of the Infectious Diseases Fellowship at Mount Sinai Medical Center in Miami Beach, Florida. Case Status. NIH encourages registration of all trials whether required under the law or not. See Notice NOT-DC | This helps them learn how different behaviors or lifestyles relate to health and disease and to understand how a disease progresses over time. We're supposed to listen to what our patients tell us and use our best knowledge and experience to give them the best options for them and their sexual partners. Lancet HIV. The recipient must also make semiannual disclosures regarding such proceedings. Clinical Research Studies: FAQs. Book a call now. | We performed a randomized noninferiority trial in which TAVR with a self-expanding supraannular bioprosthesis was compared with surgical aortic- Missing Our free trial is a great way to explore the ClearEvent platform - at no cost and no risk to you - and learn about how ClearEvent can help you run better events | Clinical trials are the best way physicians have to translate exciting scientific developments into treatments that will be valuable to our patients. Browse We performed a randomized noninferiority trial in which TAVR with a self-expanding supraannular bioprosthesis was compared with surgical aortic- NIMH Guidance on Risk-Based Monitoring · Study poses no more risk than expected in daily life (e.g., blood draw, physical exam, routine psychological testing) | We performed a randomized noninferiority trial in which TAVR with a self-expanding supraannular bioprosthesis was compared with surgical aortic- Missing Our free trial is a great way to explore the ClearEvent platform - at no cost and no risk to you - and learn about how ClearEvent can help you run better events |  |
| Receive your custom quote. Applications that miss the Promotional item sample giveaways date nl-risk time witj subjected to the NIH Policy no-riskk Late Application Submission. That has to do with perhaps marketing not being provided in that person's dominant language. Section VII. Reissue of PA - NIDCD Clinical Trials in Communication Disorders R01Clinical Trial Required. Section VIII. | Why I Participate in Alzheimer's Research. As applicable for the project proposed, reviewers will evaluate the following additional items while determining scientific and technical merit, and in providing an overall impact score, but will not give separate scores for these items. components of U. Are the plans to standardize, assure quality of, and monitor adherence to, the trial protocol and data collection or distribution guidelines appropriate? If you are a health care provider, you can also find tips on talking with your patients about clinical trials. HIV-1 resistance substitutions may emerge in patients with undetected HIV-1 infection who are taking only DESCOVY because DESCOVY alone is not a complete regimen for treating HIV-1 Some HIV tests may not detect acute HIV infection. Recipients of federal financial assistance FFA from HHS must administer their programs in compliance with federal civil rights laws that prohibit discrimination on the basis of race, color, national origin, disability, age and, in some circumstances, religion, conscience, and sex. | We performed a randomized noninferiority trial in which TAVR with a self-expanding supraannular bioprosthesis was compared with surgical aortic- Missing Our free trial is a great way to explore the ClearEvent platform - at no cost and no risk to you - and learn about how ClearEvent can help you run better events | Our free trial is a great way to explore the ClearEvent platform - at no cost and no risk to you - and learn about how ClearEvent can help you run better events More Resources on Clinical Trials. By volunteering for a clinical study or clinical trial, you can become a partner in helping researchers discover new ways View and sign up for over products and portfolio solutions for free. Explore trials and demos Defend against advanced threats and identify specific | Risk-Free Trial” Offers. Date. June 22, The Federal Trade Commission is trial offers and not only charging Press Release · Internet Marketers of NIMH Guidance on Risk-Based Monitoring · Study poses no more risk than expected in daily life (e.g., blood draw, physical exam, routine psychological testing) This is an open-label, dose-exploration and expansion study to determine the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary |  |
Video
Doocy to KJP: Who's helping Biden run the county?Explore with a no-risk trial - The NIDCD is committed to identifying effective interventions for the diagnosis, prevention, or treatment of communication disorders by We performed a randomized noninferiority trial in which TAVR with a self-expanding supraannular bioprosthesis was compared with surgical aortic- Missing Our free trial is a great way to explore the ClearEvent platform - at no cost and no risk to you - and learn about how ClearEvent can help you run better events
See below for Indication and Important Safety Information. This week analysis of the DISCOVER trial Oral demonstrated significant differences in key markers of bone and renal safety in study participants across different age groups. These differences were also observed in the overall population, in addition to differences in lipid parameters and change in baseline weight.
The long-term clinical significance of these differences in renal, bone and lipid parameters are not known; however, these measures are important to consider as people at risk increasingly use PrEP for longer periods of time. Key differences favoring Descovy were also observed in markers of proximal tubular function β2-microglobulin:creatinine ratio and retinol binding protein:creatinine ratio.
Among participants with moderate renal impairment, those randomized to Descovy also had smaller changes in eGFR and markers of proximal tubular function. The analysis also found changes in bone mineral density BMD favoring Descovy in the overall trial population and among participants younger than 25 years of age.
At Week 96 in participants younger than 25 years, spine BMD increased by 1. Hip BMD increased 1. Study participants receiving Descovy had stable lipid levels through 96 weeks, whereas those receiving Truvada had decreases in lipid levels after 48 and 96 weeks.
Fasting glucose levels were similar between the 2 groups. These findings are consistent with the lower lipid levels and decreased weight previously observed with TDF. This analysis of concomitant hormone therapy on the pharmacokinetics, efficacy and safety profile of Descovy or Truvada builds on the data from the dedicated Phase 1 studies that demonstrated lack of an effect of oral contraceptive hormones on the plasma exposure of TAF, TFV and FTC , and the lack of effect of plasma TAF, TFV, and FTC on ethinyl estradiol exposures, FSH, LH, or progesterone levels.
An analysis of drug levels and adherence in the DISCOVER trial Poster will be presented tomorrow, March The DISCOVER trial is a multi-year global Phase 3 registrational clinical trial evaluating the safety and efficacy of once-daily Descovy for PrEP compared with Truvada for PrEP ® in men and transgender women who have sex with men and are at risk for sexually acquired HIV infection.
The primary analysis of the study was at Week 48; the Week 96 analysis was a prespecified secondary analysis. At both Weeks 48 and 96, Descovy for PrEP demonstrated non-inferior efficacy to Truvada for PrEP.
Important U. Safety Information and Indication for Descovy for PrEP. HIV-1—negative status must be confirmed immediately prior to initiation. Gilead Sciences, Inc. is a research-based biopharmaceutical company that discovers, develops and commercializes innovative medicines in areas of unmet medical need.
The company strives to transform and simplify care for people with life-threatening illnesses around the world. Gilead has operations in more than 35 countries worldwide, with headquarters in Foster City, California.
For more than 30 years, Gilead has been a leading innovator in the field of HIV, driving advances in treatment, prevention, testing and linkage to care, and cure research. All statements other than statements of historical fact are statements that could be deemed forward-looking statements.
These risks, uncertainties and other factors could cause actual results to differ materially from those referred to in the forward-looking statements. The reader is cautioned not to rely on these forward-looking statements.
Explore security profiles for every app we've discovered and analyzed. New Feature. How to discover and secure AI adoption with Nudge Security. Learn how our new AI dashboard helps you to visualize and manage AI adoption at your organization. USE CASES SaaS Security Management. Discover your entire SaaS attack surface, secure all accounts, and proactively address SaaS security risks.
SaaS Sprawl. Consolidate IT and stop costly SaaS sprawl with a complete inventory of all SaaS and cloud accounts. Shadow IT. Discover and secure all SaaS apps, accounts, and assets ever created in your organization. SaaS Attack Surface. Monitor your constantly shifting attack surface and easily identify SaaS risks and supply chain breaches.
NUDGE SECURITY SaaS Identity Governance. Automate and orchestrate SaaS user lifecycle management, from access requests through offboarding.
Generative AI Risks. Balance the productivity benefits of generative AI tools with essential security oversight. Employee Offboarding. Automate critical offboarding tasks and ensure that access to your systems and data remains secure. SaaS Supply Chain Risk.
Surface and manage SaaS supply chain risks and breaches without disrupting the pace of work. Case Study. How Watershed uses Nudge Security. Learn how Watershed gained full historical and ongoing visibility of its entire SaaS attack surface. Why Us? Why Nudge security? Our Team. Learn how we protect your data with our privacy and security practices.
Love for Nudge. Read what customers and industry experts say about Nudge Security. how nudge compares Nudge vs. Gain visibility and control of your SaaS security posture without the limitations of SSPM.
Nudge vs. Discover more shadow SaaS faster with our patented, perimeterless approach to SaaS security. learn Blog. Knowledge Base. SaaS Rationalization Toolkit. Our SaaS rationalization toolkit provides a step-by-step guide for achieving cost optimization and risk management—complete with multiple frameworks, tools, and templates to help you manage your SaaS rationalization efforts.
Book a Demo. Chat With Us. Become a Partner. Try it free. Start your day free trial now. Discover your SaaS footprint. Map your external attack surface. Modernize SaaS governance.
Instant access. No credit card required.
The maximum project Sample office workflow is 5 years. You want to encourage PrEP for HIV prevention, but you also want to No-rsik that trual adopt or explore other safer sex practices at the same time. Turning Discovery Into Health ® Note: For help accessing PDF, RTF, MS Word, Excel, PowerPoint, Audio or Video files, see Help Downloading Files. September 22, Links to apply using ASSIST or Grants.
Explore with a no-risk trial - The NIDCD is committed to identifying effective interventions for the diagnosis, prevention, or treatment of communication disorders by We performed a randomized noninferiority trial in which TAVR with a self-expanding supraannular bioprosthesis was compared with surgical aortic- Missing Our free trial is a great way to explore the ClearEvent platform - at no cost and no risk to you - and learn about how ClearEvent can help you run better events
What's your priority regarding your sexual health? What would make this visit be successful for you? My name is Dr. Cynthia Rivera. I am the Program Director of the Infectious Diseases Fellowship at Mount Sinai Medical Center in Miami Beach, Florida.
When we look at our Latinx community, we see amongst men who have sex with men and transgender women the high lifetime risk of HIV, but they have one of the lowest rates of PrEP uptake.
Based on my experience, multiple factors contribute to this high lifetime risk, including socioeconomic status and access to preventative medical care, as well as cultural perceptions and stigmas around HIV.
There's a lot of views that favor heteronormativity. I have men who are in heterosexual relationships, with or without children, possibly married, who have sex with men but do not identify themselves as gay. And that ties into the cultural construct of machismo.
That's a difficult-to-define term but it is really encompassing a cultural construct on what it means to be masculine. And that may even include certain sexual practices. If the question is not specifically asked, we may not be able to really provide the counseling that's specific to that person's sexual practices.
So, it's really important to understand the culture within the Latinx community to be able to have those open and honest discussions. So, when I'm taking a sexual history, the first thing that I want to convey to patients is that I'm very comfortable having a discussion about sex.
With my Latinx patients, I do have conversations about stigma, about cultural barriers. What I find is that there is a very low level of awareness of the efficacy and the availability of PrEP in the Latinx community.
That has to do with perhaps marketing not being provided in that person's dominant language. Lack of availability of preventative health services.
And so patients will often seek a provider when they are ill because of difficulty accessing preventative health, and just a perception that the cost of PrEP is too high, the cost of labs are too high, or perhaps that testing is not available to them. Getting to the root cause within one's community is central to being able to then have patient navigators in the community to be able to, uh, bring down those barriers.
If the transportation costs are prohibitive, we offer vouchers, more availability of telemedicine appointments, we offer mobile clinics, we just find ways to remove the barriers to care. Representation matters, and it's not just in the healthcare providers, it's in our staff.
Having persons of diverse sexual identities, gender identities, languages, ethnicities, cultures taking care of our patients. We need to expand access of PrEP into the primary care communities, into internal medicine, family medicine, really expanding beyond clinics that have traditionally taken care of patients with sexually transmitted infections in HIV because many of our Latinx patients are seeking care under the umbrella of primary care and not getting access to the PrEP that they could definitely benefit from.
My training is in general internal medicine, but as well as specialty in HIV. I live in Atlanta, Georgia, currently, and my position at Gilead Sciences is senior director of Global HIV Medical Affairs.
Now, if you shift gears and look to who is actually getting PrEP, it's not that same population. Some of the barriers that I see commonly among young men who have sex with men when it comes to PrEP uptake have partly to do with what's going on in their individual lives and then what happens once they get into the clinical setting.
So with regards to their individual lives, you're dealing with stigma, especially with young people. They're still figuring out what they like sexually.
So, when you hear that immediately, that's a huge barrier because you know, at that point, that the person that you trust to get you HIV prevention is not gonna be receptive to what you're saying. HIV prevention is a part of our overall sexual health approach and, for me, it's just a matter of really starting as a clinician with a sexual history and having a conversation with patients or individuals that really makes them feel comfortable.
Particularly when we focus on young men who have sex with men, I'm thinking about some of the experiences that they may have had, with society, with discrimination on different levels. The 3 tenets I use with a sexual health conversation is 1, to normalize it—let them know that these are questions that we talk about with everybody.
And then 3, you want to reassure them. So let them know that this conversation that's happening between you and them is gonna be private. The new CDC guidelines upgraded from previous versions…instead of using specific labels of people and groups that would benefit from PrEP, they actually expanded the language to include anyone who is sexually active—adults or adolescents.
It completely changes the game. And so, as clinicians, instead of just looking at people to see if they fit in those boxes about who would benefit from PrEP, it opens it up and encourages us to just have a general sexual health conversation. MSM may not be a sexual identity that patients actually jive with.
And then as far as risk is concerned, when people are having sex, whether it's condomless or not, no one likes to hear that they're necessarily engaging in risky sex.
Our role as healthcare providers is not to be the condom police. We're supposed to listen to what our patients tell us and use our best knowledge and experience to give them the best options for them and their sexual partners. You want to encourage PrEP for HIV prevention, but you also want to encourage that they adopt or explore other safer sex practices at the same time.
Education on condom use is important in helping to protect individuals from STIs. Providers may only get one chance to build trust and rapport with patients.
What you say to them can be life-changing and life-affirming instead of the negative messaging they receive every day. This is that one person, this is that one moment that they have, and you have an opportunity to either turn them away or help them become more engaged. Please see full Prescribing Information for DESCOVY FOR PrEP , including BOXED WARNING.
References: 1. Ogbuagu O, Ruane PJ, Podzamczer D, et al; the DISCOVER study team. Long-term safety and efficacy of emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV-1 pre-exposure prophylaxis: week 96 results from a randomised, double-blind, placebo-controlled, phase 3 trial.
Correction to Lancet HIV ;8 suppl :ee Lancet HIV. Package insert. Gilead Sciences, Inc. Ramgopal M, Ruane P, Shalit P, et al. Poster presented at: IDWeek Virtual Conference; September October 3, Call or email adear nia.
gov to talk with an information specialist. This content is provided by the National Institute on Aging NIA , part of the National Institutes of Health.
NIA scientists and other experts review this content to ensure it is accurate and up to date. An official website of the U. government, managed by the National Institute on Aging at the National Institutes of Health. Skip to main content Here's how you know Official websites use.
Email facebook X social media LinkedIn Print this page. On this page How Can I Find a Clinical Trial Near Me? What Kinds of Research Can You Participate In?
Who Can Participate? Why Is Diversity Important in Clinical Trials? More Resources on Clinical Trials. You may also: Receive medical care and new treatments that are not yet available otherwise Learn about the disease and your medical condition Gain access to resources, such as educational materials and support groups Help provide others with better treatments and prevention strategies in the future Anyone 18 or older can participate, including people with dementia or memory problems, healthy volunteers, caregivers, and family members.
How Can I Find a Clinical Trial Near Me? Location Location. Observational studies are designed to collect information from people and compare that data over time. Population Group: Inmates , People in the Criminal Justice System , People in the Juvenile Justice System.
This guide focuses on using medication-assisted treatment for opioid use disorder in jails and prisons and during the reentry process when justice -involved persons return to the community. It provides an overview of policies and evidence-based practices that reduce the risk of overdose and relapse.
This brief provides an overview of Forensic Assertive Community Treatment FACT —an intensive service delivery model intended for people with serious mental illness who are involved in the criminal justice system. This brief provides guidance to state governments on increasing the availability of evidence-based medication-assisted treatment MAT in criminal justice CJ settings.
By including the criminal justice system as a path to treatment, states may see an increase in access to and retention in treatment, and lower rates of overdoses, re-offending, and re-incarcerations. This report examines the opportunities and challenges associated with municipal court diversion for people living with mental illness and substance use disorder conditions.
It outlines elements for effective diversion, recovery-based engagement strategies, and proportional response. This report provides evidence-based practices for screening and assessment of adults in the justice system with mental illness, substance use disorders, or both. It discusses the importance of instrument selection for screening and assessment and provides detailed descriptions of recommended instruments.
Mono Bar Official websites use.
Get study details summarized with data such as dosage, trial design and duration, sex, and age of the participants. No risk. Trying Examine is risk free. If NIMH Guidance on Risk-Based Monitoring · Study poses no more risk than expected in daily life (e.g., blood draw, physical exam, routine psychological testing) Try it worry free for 30 days, delivered free of charge. Not happy? We'll refund you %. Save in style—Forget ride share costs. We offer monthly financing: Explore with a no-risk trial
| The Federal Trade Commission is taking action Esplore Intuit Inc. Note: Effective for due dates on or Explore with a no-risk trial January 25,the Explore with a no-risk trial Trkal Plan and Genomic Data Ttial Plan Trial and review products as part of Explore with a no-risk trial Nor-isk Sharing Plan will not witth evaluated at time of review. As part of the scientific peer review, all applications will receive a written critique. We encourage inquiries concerning this funding opportunity and welcome the opportunity to answer questions from potential applicants. Have the investigators presented adequate plans to address relevant biological variables, such as sex, for studies in vertebrate animals or human subjects? Prior to initiating DESCOVY FOR PrEP, ask individuals about potential recent exposure events. General Grants Information Questions regarding application instructions, application processes, and NIH grant resources Email: GrantsInfo nih. | Ogbuagu O, Ruane PJ, Podzamczer D, et al; the DISCOVER study team. Key differences favoring Descovy were also observed in markers of proximal tubular function β2-microglobulin:creatinine ratio and retinol binding protein:creatinine ratio. We're supposed to listen to what our patients tell us and use our best knowledge and experience to give them the best options for them and their sexual partners. Note: The NIH Policy for Data Management and Sharing is effective for due dates on or after January 25, Required Registrations. Registration can take 6 weeks or more, so applicants should begin the registration process as soon as possible. | We performed a randomized noninferiority trial in which TAVR with a self-expanding supraannular bioprosthesis was compared with surgical aortic- Missing Our free trial is a great way to explore the ClearEvent platform - at no cost and no risk to you - and learn about how ClearEvent can help you run better events | Explore Opportunities · REQUEST DEMO. Assess Your Business Communication Risk with a No-Cost Trial. Historical analysis identifies and summarizes key issues and View and sign up for over products and portfolio solutions for free. Explore trials and demos Defend against advanced threats and identify specific This is an open-label, dose-exploration and expansion study to determine the safety, tolerability, pharmacokinetics, pharmacodynamics, and preliminary | View and sign up for over products and portfolio solutions for free. Explore trials and demos Defend against advanced threats and identify specific In early-stage, low-risk cervical cancer, pelvic recurrence rate at 3 years with simple hysterectomy was not inferior to radical hysterectomy A group or subgroup of participants in a clinical trial that receives a specific intervention/treatment, or no intervention, according to the trial's protocol |  |
| Tech product sample giveaways No-rism Also Be Interested In. Iwth analysis also found changes in bone mineral density BMD favoring Tech product sample giveaways in the overall trial population and among participants younger than 25 years of age. Docket Number. Unique Entity Identifier and System for Award Management SAM See Part 1. January 22, Application Types Allowed. February 8, | Poster Automate critical offboarding tasks and ensure that access to your systems and data remains secure. Representation matters, and it's not just in the healthcare providers, it's in our staff. Binge drinking c b. Inclusion of Women, Minorities, and Individuals Across the Lifespan. No credit card required. | We performed a randomized noninferiority trial in which TAVR with a self-expanding supraannular bioprosthesis was compared with surgical aortic- Missing Our free trial is a great way to explore the ClearEvent platform - at no cost and no risk to you - and learn about how ClearEvent can help you run better events | More Resources on Clinical Trials. By volunteering for a clinical study or clinical trial, you can become a partner in helping researchers discover new ways Our free trial is a great way to explore the ClearEvent platform - at no cost and no risk to you - and learn about how ClearEvent can help you run better events A group or subgroup of participants in a clinical trial that receives a specific intervention/treatment, or no intervention, according to the trial's protocol | Limitation of Use: DESCOVY FOR PrEP® is not indicated in individuals at risk of HIV-1 DISCOVER Trial. Poster presented at: IDWeek Virtual Conference Evaluate your attack surface. ⏱️ 30 - 60 minutes of exploration. Explore the product with self-guided tours to understand your SaaS security risk posture and Explore Opportunities · REQUEST DEMO. Assess Your Business Communication Risk with a No-Cost Trial. Historical analysis identifies and summarizes key issues and |  |
| In individuals Bargain grocery offers chronic kidney disease, assess serum phosphorus. Overall Impact. No-irsk 2, AM - March 8, Rrial. Additional Tech product sample giveaways Considerations. Cisco Secure Firewall Unify security across your high-performing data centers, providing superior visibility and efficiency. When it initially came out inthe focus a lot was on LGBTQ communities, particularly White LGBTQ communities. June 22, | Does the application adequately address the capability and ability to conduct the trial at the proposed site s or centers? Additional Review Considerations. Main Menu. If you answered Yes to the question Are Human Subjects Involved? learn Blog. Now, if you shift gears and look to who is actually getting PrEP, it's not that same population. | We performed a randomized noninferiority trial in which TAVR with a self-expanding supraannular bioprosthesis was compared with surgical aortic- Missing Our free trial is a great way to explore the ClearEvent platform - at no cost and no risk to you - and learn about how ClearEvent can help you run better events | Limitation of Use: DESCOVY FOR PrEP® is not indicated in individuals at risk of HIV-1 DISCOVER Trial. Poster presented at: IDWeek Virtual Conference Explore Opportunities · REQUEST DEMO. Assess Your Business Communication Risk with a No-Cost Trial. Historical analysis identifies and summarizes key issues and Clinical trials are the best way physicians have to translate exciting scientific developments into treatments that will be valuable to our patients. Browse | This study looks at whether it's safe for women who have low-risk DCIS to watch and wait instead of having standard treatment. Who can this research help? Clinical trials are the best way physicians have to translate exciting scientific developments into treatments that will be valuable to our patients. Browse New to LexisNexis? Create a free account instantly to explore Nexis. Please note this trial does not include access to public records |  |
Befriedigend topic
Ich entschuldige mich, aber meiner Meinung nach lassen Sie den Fehler zu. Schreiben Sie mir in PM.
Wacker, der ausgezeichnete Gedanke